Which of the Following Atoms Has the Greatest Nuclear Charge
Considering periodic trends valence electrons in which of the following atoms experience the greatest effective nuclear charge Zeff. 2 on a question Which of the following atoms has the greatest nuclear charge.

A Timeline Of Atomic Models Chemistry Lessons Atom Teaching Chemistry
Which one of the following ions has a smallest radius.
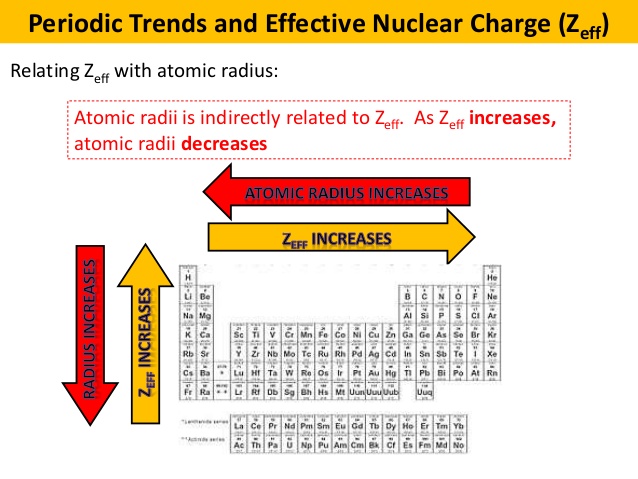
. Science Chemistry QA Library Considering periodic trends valence electrons in which of the following atoms experience the greatest effective nuclear charge Zeff. Which of the following statements best describes how atom X and atom Y are related. Up to 10 cash back Because chlorine is in the same period as phosphorus and sodium but has the most protons in its shell the most right within the same period it has the greatest effective nuclear charge.
They are the same distance in all of these atoms. Additionally because chlorine is in the same group as bromine but is higher up on the periodic table it has a greater effective nuclear charge making it the correct. Which of the following has the greatest nuclear charge.
Whitch of the following atoms has the greatest nuclear charge. Categories Question-Answer Leave a Reply Cancel reply. Atom X has 9 protons 10 neutrons and 9 electrons.
Higher the number of electrons in the extranuclear part Be has 4 Be2 has 2 while Be3 has 1 lower is the force of attraction of the nucleus on the valence electrons and hence lower is the effective nuclear charge. Thus Be has minimum effective nuclear charge ie option a is correct. Cl is the largest and has the greatest effective nuclear charge.
A CI в С C F D Ne E B. Because K has the greatest nuclear charge Z 19 its radius is smallest and S 2 with Z 16 has the largest radius. Li Be Ba F.
Whitch of the following atoms has the greatest nuclear charge. Solution for Which one of the following atoms has the largest effective nuclear charge Zeff. The effective nuclear charge experienced by electrons in an atom is a function of the distance of the electrons from the nucleus of the atom.
Chemistry questions and answers. In essence the effective nuclear charge on electrons increases. Atoms can be considered the basic building blocks of matter.
N-14 C-12 H-2 He-4. A Al B At C Si D Na. What is the nuclear charge of an atom with a mass of 23 and atomic number of 11.
In which of the following atoms is the 2s orbital closest to the nucleus. A CI BC CF D Ne E B. The arrangement of the atoms in order of increasing effective nuclear charge experienced by the electrons in the n3 electron shell is.
Rank the following atoms in order of decreasing first ionization energies ie highest to lowest. Finally Which of the following atoms has the greatest nuclear charge charge of the nucleus Because chlorine is in the same period as phosphorus and sodium but has the most protons in its shell the most right within the same period. Bonus question worth 2 marks answer choices.
11 all atoms in a given sample of an element contain the same number of. Which of the following atoms has the greatest nuclear charge. 2 October 2021 by lets tokmak.
As argon has more number of. 4 rows Which of the following atoms has the greatest nuclear charge. Considering periodic trends valence electrons in which of the following atoms experience the greatest effective nuclear charge zeff.
IThe Zeff for the highest energy electron in Be is higher than the Zeff for the highest energy electron for NIIThe second ionization energy for Mg is greater than the first ionization energy of NaIIIThe species O2-. Considering periodic trends valence electrons in which of the following atoms experience the greatest effective nuclear charge Zeff. Rh Ti K Mg P.
Given the three statements below pick the best answer. Atom Y has 9 protons 9 neutrons and 9 electrons. Whitch of the following atoms has the greatest nuclear charge.
Calcium comes the last and forms the smallest ion.

Tetryonics 54 11 Modern Nuclear Isotope Theory Represents One Of The Greatest Foundational Errors Of Assumption In Mode Nuclear Energy Elements Quantum Leap

No comments for "Which of the Following Atoms Has the Greatest Nuclear Charge"
Post a Comment